
March 02, 2022
 Courtesy/Thomas Jefferson University
Courtesy/Thomas Jefferson University
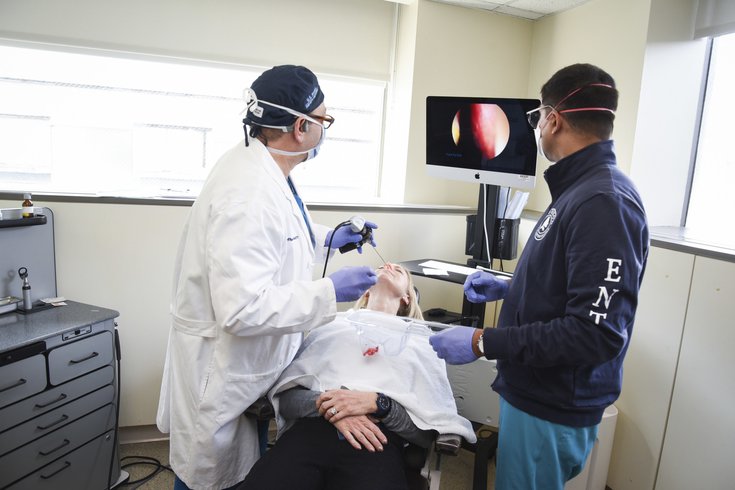
Dr. David Rosen, left, uses an endoscope to place a topical platelet-rich plasma in Nancy Damato's nose as Dr. Glen D'Souza watches. The experimental treatment is aimed at restoring the loss of smell and taste.
Early data from an ongoing clinical trial led by Jefferson Health offers some hope that there may soon be a minimally invasive treatment for loss of smell and taste.
Researchers have developed a topical treatment that uses platelet-rich plasma to restore the senses.
Loss of smell and taste – known as anosmia – are common symptoms of COVID-19. Though they subside for most people infected by the coronavirus, up to 1.5 million U.S. residents continue to have long-term smell and taste disturbances, the researchers said.
Nancy A. Damato, one of the study participants, said losing her senses of smell and taste from COVID was "life changing."
"I felt like I was missing a part of myself and more than anything I missed the experience of gathering with family to enjoy a meal," she said. "Fortunately, the treatments provided by Thomas Jefferson University Hospital are improving my symptoms and showing signs of progress. For the first time in a long time, I have hope for getting my life back to normal."
Platelet-rich plasma, or PRP, can regenerate cells and heal tissue. It has become a common restorative therapy used to treat injured muscles and tendons, increase hair growth and reduce the appearance of scars.
Animal studies have shown PRP also can regenerate cells in the olfactory epithelium – thin, cellular tissue inside the nasal cavity. This tissue is thought to be the site affected by COVID-19-related loss of smell. And since smell and taste are closely linked, the hope is the treatment will improve sense of taste as well.
Some small clinical trials have tested a nasal injectable PRP for smell loss. Though the results were promising, the participants found the injections to be uncomfortable and invasive.
Jefferson Health's topical PRP is considered an less invasive option. The PRP is mixed with a dissolving sponge and applied to the olfactory nerve in the nose via an endoscope. It is a painless procedure.
In a phase I trial, eight patients who had at least six months of olfactory disturbances received monthly applications of the topical PRP for at least three months. Preliminary results show that 50% of the participants experienced significant improvements in smell and taste.
The new treatment also has been provided to additional patients independent of the trial with similarly promising results.
In a phase II study, the researchers plan to look exclusively at patients who developed long-term loss of taste and smell after a COVID-19 infection. This will help them better understand patient variables and the number of treatments needed to provide the most significant improvement.
"It was very important to me and our team to explore less invasive options as this issue has become increasingly prevalent due to COVID-19," said Dr. David Rosen, an otolaryngologist at Thomas Jefferson University Hospital. "The results of phase I of the clinical trial have been promising and we are looking forward to phase II to further improve the treatment."
The treatment is not covered by insurance and costs $500 per application, but it will be available to patients enrolled in the next phase of the clinical study free of charge.