Penn Medicine will become the first hospital in the Northeast to conduct a clinical trial for uterine transplantation, an experimental procedure that could provide a new path to parenthood for women with uterine factor infertility.
UFI, an irreversible form of female infertility, impacts about 50,000 women in the United States and 5 percent of women worldwide. Affected women are unable to carry a pregnancy either because they were born without a uterus, their uterus was surgically removed or their uterus doesn't function properly.
- RELATED ARTICLES
- Penn study finds women with infertility face higher rate of cancer-related death
- Study: You're officially not supposed to eat your placenta
- BINTO brings fertility products and knowledge to women's doorsteps
The Uterine Transplantation for Uterine Factor Infertility (UNTIL) trial, the third of its kind in the United States, aims to build on the success of international efforts that have shown promise in recent years.
More than 30 uterine transplants have been completed in Brazil, China, the Czech Republic, Germany, Sweden, India, Saudi Arabia, Turkey, and at two clinics in the United States. In 2014, a Swedish woman gave birth to the world's first child from a uterine transplant performed by a team at the University of Gothenburg, which has since delivered seven other babies.
“While there are still many unknowns about uterine transplantation, we know this approach has the potential to give women with UFI a real opportunity to carry and deliver a child,” said Kate O’Neill, an assistant professor of Obstetrics and Gynecology in the Perelman School of Medicine. “The strength of Penn’s research program and exceptional quality of care, particularly in transplant and women’s health, makes us uniquely positioned to be a leading clinical research program for uterine transplants in the United States, and to continue to advance this emerging field.”
Participants in the trial will undergo a multiyear process during which they'll be followed by specialists in various disciplines, from pathology and infectious diseases to psychology, bioethics and nursing. A pre-transplant phase will involve in vitro fertilization to harvest the patients' eggs, followed by a transplant evaluation, surgery and immunosuppressant medication.
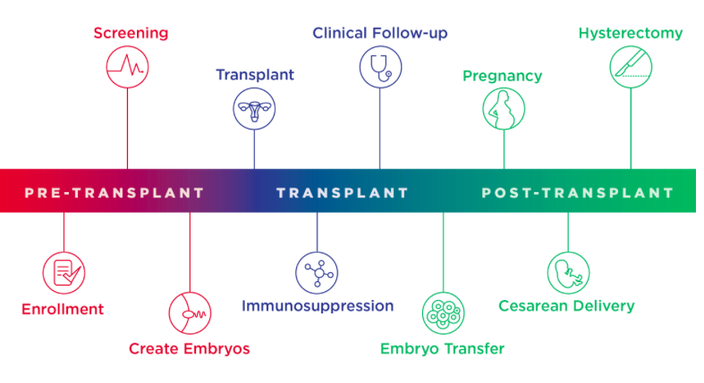
“In most cases, the absence of a uterus also means these women likely do not have a ‘normal’ vaginal canal, making it risky, or impossible, for them to have a vaginal delivery. However, our team of experts is taking every precaution to ensure the health and safety of both our mothers and babies,” said Eileen Wang, who will join O'Neill and transplant surgeon Paige Porrett as co-principal investigators on the UNTIL trial.
Organs for the trial, procured through the Gift of Life Donor program, will be transplanted from deceased women between 21 and 40 years old who have successfully delivered healthy babies. Once women participating in the trial have delivered up to two healthy babies, the transplanted uterus will be removed via hysterectomy so that they can discontinue anti-rejection medication.
The team at Penn has spent the past year practicing the transplant surgery at the university's Human Tissue Lab, which has helped spearhead recent growth in the field of vascularized composite allotransplantation (VCA), which encompasses the procedure used in the UNTIL Trial.
“The results of the VCA surgeries performed here at Penn have been very promising, and give us the foundation necessary to continue moving ahead with advancements in the field,” Porrett said. “Leadership from OB-GYN and transplant have been incredibly supportive of our mission to help more women and their families realize their dreams of becoming pregnant, carrying healthy babies and becoming parents.”

