March 06, 2019
The U.S. Food and Drug Administration has announced a nationwide birth control recall as a result of a packaging error.
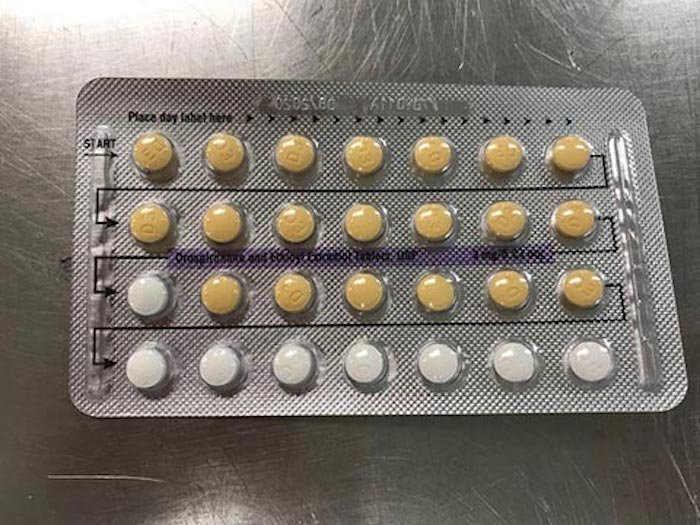
Apotex Corporation has recalled four lots of Drospirenone and Ethinyl Estradiol birth control pills because the tablets may have been arranged incorrectly or could be missing, the FDA announced Monday.
Because of the error, women may find themselves mistakingly missing a pill or taking a placebo instead of an active pill, which can result in pregnancy. “As a result of this packaging error, where a patient does not take a tablet due to a missing tablet or that a patient takes a placebo instead of an active tablet, loss of efficacy is possible due to variation in the dosage consumed,” the FDA recall reads.
RELATED READ: FDA: Two retailers for youths sold makeup containing asbestos
As of Monday, there have been no reports of any adverse effects or unintended pregnancies, according to the FDA.
A list of the affected lots, which all have an expiration date of August 2020, can be reviewed here. View an incorrectly arranged Apotex birth control pack below:
“Individuals should not interrupt their therapy, use a non-hormonal method of birth control, contact their health care provider for medical advice and may return the impacted packages to their pharmacist,” the FDA said in a statement.
Apotex has also notified places where the pills were shipped and has arranged for them to be returned.