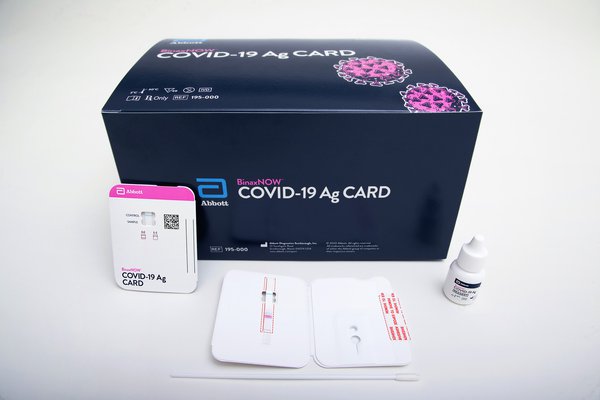
The U.S. Food and Drug Administration has authorized a COVID-19 test that produces a rapid result using the same technology as pregnancy tests.
The BinaxNOW test, manufactured by Abbott Diagnostics Scarborough Inc., only costs $5 and produces results within 15 minutes. It's the first coronavirus test where results can be read directly from a testing card.
- MORE HEALTH
- Rapid screening tests may be key to ending the coronavirus pandemic
- CHOP researchers see sense of altruism in people's COVID-19 worries
- Blood plasma authorized as COVID-19 treatment, but some question decision
- Swab, spit, stay home? College COVID-19 testing plans are all over the map
A health care provider first swabs the patient’s nose and then twirls the sample on the card with a testing regent added. If one line appears on the card, the test result is negative. Two lines indicate a positive test result.
The FDA's emergency use authorization requires health care providers to administer the test. It can be conducted at approved point-of-care locations on patients suspected of COVID-19 within seven days of symptom onset.
The test relies on antigens to determine its results, unlike the molecular tests that detect the virus's genetic material. Antigen tests produce faster results, but they generally are not as accurate as molecular tests.
Because of the decreased sensitivity, patients who receive negative test results may need to confirm those results via a molecular test, the FDA said. But the advantage of antigen tests is that they catch positive cases faster, potentially preventing additional people from being exposed to the virus.
According to Abbott, the test accurately diagnoses a positive case 97.1% of the time and correctly diagnoses a negative case 98.5% of the time.
"This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card," said Dr. Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health.
"This means people will know if they have the virus in almost real-time. Due to its simpler design and the large number of tests the company anticipates making in the coming months, this new antigen test is an important advancement in our fight against the pandemic."
Abbott is currently shipping out about 1 million tests per day. By October, the manufacturer intends to make 50 million tests available on a monthly basis.
The company said it has invested hundreds of millions of dollars into two new facilities to scale up the BinaxNOW test.
Abbott also has developed a corresponding mobile app, Navica, which allows people to store, access and display their test results with organizations that accept results.
If test results are negative, the app will display a digital health pass via a QR code. If test results are positive, patients receive a message to self-quarantine and contact their doctor.
"We intentionally designed the BinaxNOW test and Navica app so we could offer a comprehensive testing solution to help Americans feel more confident about their health and lives," Abbott CEO Robert B. Ford said. "BinaxNOW and the Navica app give us an affordable, easy-to-use, scalable test and a complementary digital health tool to help us have a bit more normalcy in our daily lives."
This is the sixth test that Abbott has launched in the U.S. during the COVID-19 pandemic. The company has provided more than 27 million COVID-19 diagnostics to date, including 14 million detection tests and 13 million antibody tests.
Follow Pat & PhillyVoice on Twitter: @Pat_Ralph | @thePhillyVoice
Like us on Facebook: PhillyVoice
Add Pat's RSS feed to your feed reader
Have a news tip? Let us know.